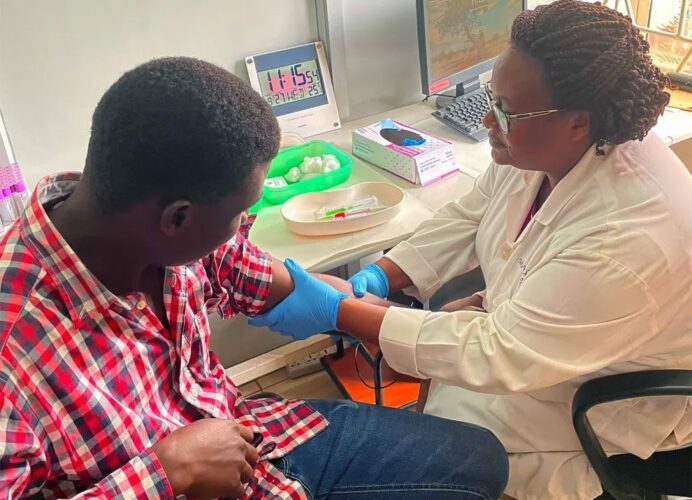
Healthy volunteers today received a single-dose vaccine for Marburg virus as part of a Phase 2 clinical trial at Makerere University Walter Reed Project (MUWRP) in Kampala, Uganda.
Participants will also be enrolled a few weeks later at a second site at the Kenya Medical Research Institute in Siaya, Kenya. In all, 125 volunteers will participate in the trial.
Based on the ChAd3 platform, Sabin Vaccine Institute’s single-dose investigational Marburg vaccine was found to be promising in Phase 1 clinical and non-clinical studies, with results showing it to be safe, while eliciting rapid and robust immune responses.
There are currently no vaccines or antiviral treatments approved to treat Marburg virus disease. Marburg is a filovirus, in the same family as the virus that causes Ebola. Like Ebola, Marburg virus disease spreads between people via direct contact with the blood or other bodily fluids of infected people, is highly virulent, and causes hemorrhagic fever. The disease has a fatality rate of up to 88%.
The number of Marburg outbreaks in Africa has climbed steadily in recent years. Two outbreaks of Marburg virus disease have occurred already this year: Equatorial Guinea reported its first ever documented Marburg outbreak, which killed 12 people, followed by Tanzania, where six people succumbed to the virus. Communities in Uganda and Kenya are familiar with Marburg virus disease, having been ravaged by outbreaks over multiple years in the last few decades.
“We have an extraordinary opportunity here to improve our preparedness to save lives and protect people from a deadly and unforgiving disease that typically strikes under-resourced countries first and most. Sabin’s Phase 2 clinical trial builds on a solid safety and immunogenicity foundation and we are hoping it will generate the information needed to move the vaccine toward licensure.”
Amy Finan, Sabin’s Chief Executive Officer
The Phase 2 clinical trial for Sabin’s Marburg vaccine will continue to evaluate safety and immunogenicity for the vaccine, this time among a larger group of individuals. This is a randomized, placebo-controlled, double-blind study, meaning that neither the participants nor the researchers will know whether trial participants receive a vaccine dose or a placebo dose until after the trial is over, an approach used to help reduce experimental bias.
Participants in the clinical trial will be monitored for a full year and will include both younger (18-50 years) and older age groups (51-70 years). Interim results are expected next year. In addition to the current trial in Uganda and Kenya, Sabin plans to conduct a similar Phase 2 clinical trial for Marburg in the U.S.
“Makerere University Walter Reed Project (MUWRP) is delighted to partner with the Sabin Vaccine Institute to launch the clinical testing for a preventive Marburg vaccine. Most Marburg virus disease outbreaks have originated in Africa. Uganda alone has registered 4 outbreaks of the disease. We urgently need a vaccine against Marburg because of its potential to cause epidemics with significant death rates. It is imperative for us to test candidate vaccines in Uganda, a country prone to these outbreaks. This work will contribute new knowledge to inform the scientific discovery for an effective vaccine against the deadly Marburg virus.”
Dr. Betty Mwesigwa, deputy executive director of MUWRP, principal investigator for the Kampala portion of the Sabin-sponsored trial
The Marburg vaccine trials are supported by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, under multi-year contracts between the organizations, including most recently a $36.4 million award for vaccine development and production.
SEE ALSO: Sabin Vaccine Institute to Advance Ebola Sudan and Marburg Vaccines with New BARDA Funding Jan 2023
Similarly, BARDA has invested in Sabin for advancing ChAd3 Sudan ebolavirus vaccine candidate, including awarding $28 million this August for Phase 2 clinical trials in the U.S.
To date, Sabin has received around $215 million in contract awards from BARDA for furthering vaccine research and development against Sudan ebolavirus and Marburg virus diseases.
BARDA and Sabin began working together in September 2019 to develop the two monovalent vaccine candidates. Sabin’s Sudan ebolavirus vaccine candidate was the first to arrive in Uganda last year during the disease outbreak that left 55 people dead. Sabin has also initiated plans for a Phase 2 Sudan ebolavirus vaccine clinical trial in Uganda and Kenya.
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA).
READ ALSO
Marburg Virus Outbreak: Researchers Race to Test Vaccines
After Equatorial Guinea confirmed its first outbreak of Marburg virus disease on 13 February 2023, health officials worldwide are sprinting to test whether experimental vaccines can protect against the deadly illness. Even if a trial can get off the ground, it’s unlikely that enough cases will develop before the current outbreak comes under control for researchers to determine conclusively whether any vaccine is effective or not. But evidence pointing to the effectiveness of any vaccine could be gathered across multiple outbreaks. Nature
Rise of Marburg Virus in Africa: A Call for Global Preparedness
The MARV has been responsible for many outbreaks since it was first discovered in 1967, with the majority of these outbreaks occurring in Africa. In 1975, the second outbreak of MVD occurred in Zimbabwe, marking the first-ever recorded outbreak of the disease in Africa. From 1975 to 1985, only a few cases of MVD were reported in Africa. Prior to 1998, MARV was not believed to be as fatal as the Ebola virus. However, this perception changed after two significant outbreaks occurred in the Democratic Republic of Congo between 1998 and 2000, and the first-ever outbreak in Angola between 2004 and 2005. Uganda experienced four epidemics in the year 2007, 2012, 2014, and 2017, each with an incident fatality percentage ranging from 27 to 100%, while between the year 1998 and 2000, the Republic of Congo reported a total of 154 cases of MVD, resulting in 128 deaths and a case-fatality rate of 83%. The first-ever MARV outbreak in West Africa was recorded in the Republic of Guinea, in August 2021. This was followed by another outbreak in Ghana in 2022, reported as the country’s first-ever case of MARV. In early 2023, Equatorial Guinea and Tanzania experienced an outbreak of MARV.
Source link : https://globalbiodefense.com/2023/10/19/phase-2-clinical-trial-for-marburg-vaccine-starts-in-uganda/
Author :
Publish date : 2023-10-19 07:00:00
Copyright for syndicated content belongs to the linked Source.